Background: Diamond- Blackfan Anemia (DBA) is a rare, heritable ribosomopathy caused by mutations in ribosomal protein large (RPL) and small (RPS) subunit genes. The diagnostic criteria of DBA include presentation in infancy with virtually no mature erythroblasts (EB) on bone marrow (BM) examination, however atypical presentations in later life with milder haematological manifestations are increasingly reported. The cellular and molecular mechanisms underpinning variable clinical phenotypes, and how they relate to genotype, are yet to be elucidated. Furthermore, as many DBA patients do not respond to corticosteroids, understanding the pathological processes contributing to erythroid failure is a prerequisite for developing novel, precision-medicine therapies.
Aim: To elucidate phenotypic and functional differences in erythropoiesis in RPS- and RPL-DBA, using primary BM samples from patients.
Methods: We performed single-cell transcriptome profiling (scRNAseq), using the 10X Genomics chromium platform, of 45,488 CD34+ Lineage negative (Lin-) BM hematopoietic stem and progenitor cells (HSPC), purified by fluorescence-activated cell sorting. We included six patients with red cell transfusion-dependent DBA (aged 2-19y) with mutations in RPS19 (n=3), RPL11 (n=1) and RPL5 (n=2) and three healthy donors (aged 3-17y). We validated our findings using bulk RNAseq, functional assays, and deep immunophenotyping-based dissection of the haematopoietic architecture of DBA BM ex vivo.
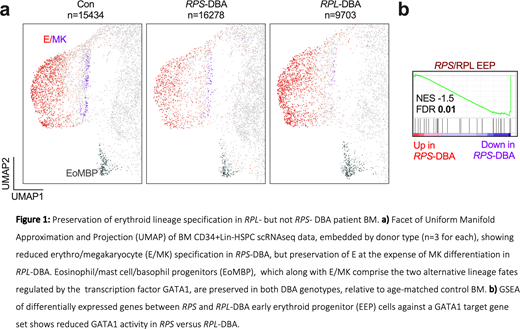
Results: High quality sequencing data was obtained for all nine donors; after quality control, 41,415 single cells were carried forward for analysis. Unsupervised clustering analysis and lineage identification revealed two divergent cellular patterns in DBA compared with age-matched control BM that segregated with genotype: a selective loss of erythromegakaryocyte (E/MK) progenitors in RPS-DBA, but relative preservation of the erythroid developmental trajectory in RPL-DBA, at the expense of megakaryopoiesis (Fig 1a).
Gene Set Enrichment Analysis (GSEA) of differentially expressed genes between control and DBA HSPC clusters revealed p53-mediated apoptosis, TNFa-, IFNa- and IFNg-mediated inflammatory pathways in DBA EP. Although these pathways were enriched across all HSPC populations irrespective of genotype, they were detected at an earlier stage of the stem cell hierarchy, and more potently, in RPS- versus RPL-EP. Expression of transcriptional targets of the master E/MK transcription factor, GATA1, was significantly upregulated in RPL- versus RPS-DBA EP (Fig 1b), supporting the maintained erythroid program in RPL-DBA HSPC. However, expression of genes denoting erythroid differentiation, including adult haemoglobin (Hb) genes, was significantly elevated in RPL-DBA, suggesting aberrant accelerated differentiation to EB.
These findings were confirmed by immunophenotypic examination of patient BM and bulk RNAseq of RPL-EB. Additionally, single-cell clonogenic assays of RPL-DBA EP showed severe qualitative defects. Thus, although erythroid commitment occurs in RPL-DBA, it is coupled with accelerated maturation beyond EP to functionally impaired mature EB, enriched in inflammatory and p53 pathways.
Finally, we analysed the clinical characteristics of the 161 patients comprising the U.K DBA registry. In line with the milder erythroid specification defect, patients with RPL-DBA (n=44) presented with anaemia later (P=0.004), and with a higher average Hb concentration (P=0.04), than those with RPS-DBA (n=63). Furthermore, we identified higher corticosteroid responses in RPL-DBA, assessed at 6 months post initiation (P=0.006), consistent with our findings in RPL-DBA of preservation of the cellular EP populations that are targeted by corticosteroids.
Impact: In conclusion, a preserved but distinct erythroid developmental trajectory, characterised by accelerated differentiation, underpins a milder haematological phenotype in RPL-DBA. Furthermore, we reveal the first single-cell transcriptomic dataset from haematopoietic cells in a ribosomopathy, uncovering novel cell intrinsic and extrinsic pathogenetic insights into failing erythropoiesis in DBA. Integration of these data with clinical genomics and phenomics provides a paradigm by which single cell approaches can be used to decipher genotype-phenotype relationships in Mendelian genetic disorders.
Mead:Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding; Abbvie: Consultancy; CTI: Consultancy; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal